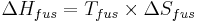Entropy of fusion
The entropy of fusion is the increase in entropy when melting a substance. This is almost always positive since the degree of disorder increases in the transition from an organized crystalline solid to the disorganized structure of a liquid; the only known exception is helium.[1] It is denoted as ΔSfus and normally expressed in J / mol · K
A natural process such as a phase change will occur when the associated change in the Gibbs free energy is negative. It follows that the entropy of fusion is related to the melting point and the heat of fusion:
Helium-3 has a negative entropy of fusion at temperatures below 0.3 K. Helium-4 also has a very slightly negative entropy of fusion below 0.8 K. This means that, at appropriate constant pressures, these substances freeze with the addition of heat.[2]
Notes
- ^ Atkins & Jones 2008, p. 236.
- ^ Ott & Boerio-Goates 2000, pp. 92–93.
References
- Atkins, Peter; Jones, Loretta (2008), Chemical Principles: The Quest for Insight (4th ed.), W. H. Freeman and Company, p. 236, ISBN 0-7167-7355-4
- Ott, J. Bevan; Boerio-Goates, Juliana (2000), Chemical Thermodynamics: Advanced Applications, Academic Press, ISBN 0-12-530985-6